In Vitro and In Vivo Characterization of BiFORM® Bioactive Moldable Matrix
Posterolateral Rabbit Spinal Fusion Study
I. Introduction
Autologous bone has long been the gold standard for bone graft substitute in spinal fusion procedures. The limited supply and morbidity associated with using autologous graft material led to the development of alternative bone grafting materials. Bioactive glass has a long history of biomedical use and has been shown to facilitate mineral deposition in vitro2,3. In order to take advantage of the surface bioactivity of bioactive glass and minimize the use of autograft, we have developed a Bioactive Glass Collagen Anorganic Bone Composite (BiFORM® Bioactive Moldable Matrix).
II. Objective
The objective of the study was to evaluate the safety and effectiveness of using BiFORM® Bioactive Moldable Matrix + BMA to facilitate fusion between L4-L5 transverse processes in a rabbit posterolateral spinal fusion model in comparison with Vitoss® BA + BMA and BiFORM® Bioactive Moldable Matrix + autograft in comparison with autograft as positive control.
III. Materials & Methods
BiFORM® Bioactive Moldable Matrix is composed of 30% 45S5 bioactive glass, 20% bovine Type I collagen, and 50% bovine anorganic bone mineral. Vitoss® BA is composed of 80% β-TCP + bioactive glass and 20% collagen.
36 New Zealand white rabbits were assigned into four study groups summarized in Figure 1.
Figure 1. Summary of Study Groups
| TEST | DESCRIPTION | DURATION | ||
|---|---|---|---|---|
| 4 Weeks | 8 Weeks | 14 Weeks | ||
| Vitoss BA + BMA | Predicate Device | 3 | 3 | 3 |
| BiFORM® Bioactive Moldable Matrix + BMA | Subject Device | 3 | 3 | 3 |
| BiFORM® Bioactive Moldable Matrix + Autograft | Subject Device | 3 | 3 | 3 |
| Autograft | Positive Control | 3 | 3 | 3 |
A single level posterolateral intertransverse process fusion was performed in a total of 36 animals, bilaterally at L4-L5 transverse processes. In 18 animals, autogenous bone graft was obtained
from the iliac crest and a combination of BiFORM® Bioactive Moldable Matrix + autograft (40:60) was used. In the remaining 18 animals, bone marrow aspirate was combined with either BiFORM® Bioactive Moldable Matrix or Vitoss® BA. At 4, 8- and 14-weeks post-surgery, rabbits were euthanized. Specimens were then harvested and prepared for histology analysis to assess the bone regeneration, fusion rate, and tissue reaction to the implant materials. Radiographic, manual palpation, histopathology and histomorphometry fusion were scored as per ASTM F3207-175.
IV. Results
BiFORM® Bioactive Moldable Matrix had approximately 2x higher fusion results than Vitoss® BA at 14 weeks in all 3 fusion assessments: Radiographic, Histopathology, and Histomorphometry, as shown
in Figure 24.
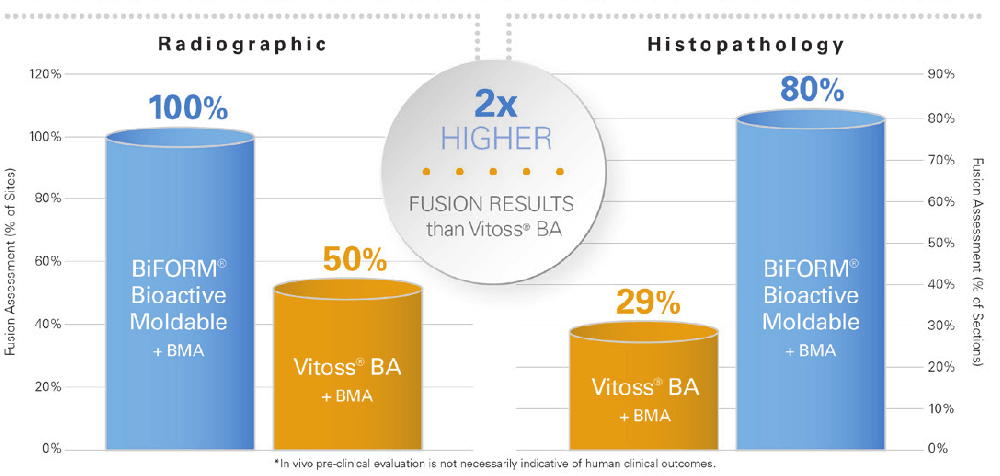
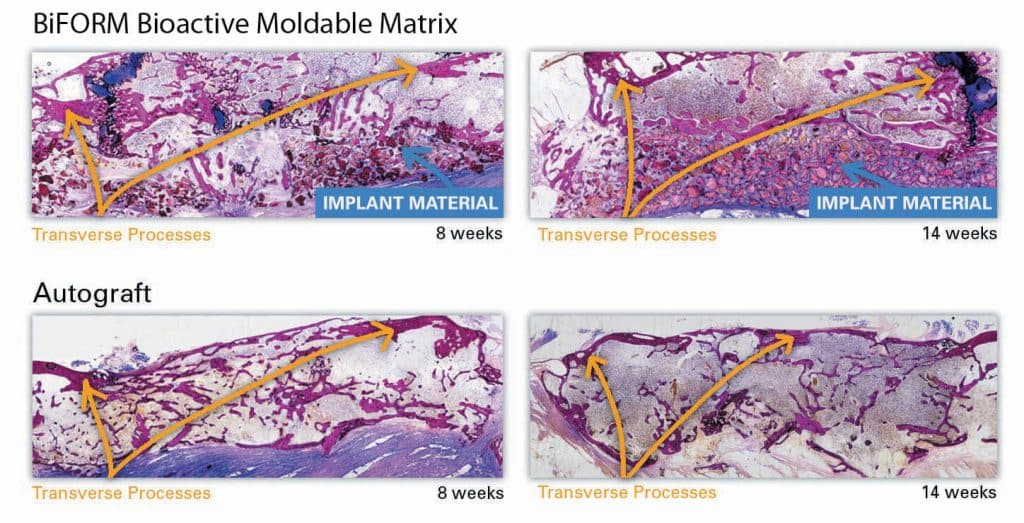
Representative histological images further confirmed that the BiFORM® Bioactive Moldable Matrix + autograft group exhibited comparable bone regeneration/retention and bridging (fusion) of the transverse processes similar to the autograft control implant sites. Most new bone growth extended across intertransverse process space as seen in Figure 3 below. No major inflammation or signs of graft rejection was evident over the course of the study.
V. Discussion
Collagen bone graft matrices are typically composed of synthetic mineral components like hydroxyapatite and β-TCP, which are intended to offer different resorption profiles. β-TCP typically resorbs away quickly, while hydroxyapatite is very slow-resorbing. Current collagen-mineral based composites on the market may attempt to mimic a “balanced” resorption profile by incorporating a combination of the two synthetic mineral components along with either a collagen or other carrier. BiFORM® Bioactive Moldable Matrix is unique in that it incorporates carbonate apatite bone mineral, which has a natural mineral structure similar to human bone mineral. Anorganic carbonate apatite bone mineral has a balanced resorption profile when compared to synthetics like β-TCP and hydroxyapatite (HA)6,7.
VI. Conclusion
The results demonstrate the performance and substantial equivalence of BiFORM® Bioactive Moldable Matrix + BMA as compared to Vitoss BA + BMA, as well as the performance and substantial equivalence of BiFORM® Bioactive Moldable Matrix + autograft as compared to the autograft control group. Both BiFORM® Bioactive Moldable Matrix (combined with BMA or combined with autograft) performed as well as or better than Vitoss® BA + BMA (Vitoss® BA predicate device was used as indicated in its instructions for use). Future studies will be conducted to evaluate the safety and effectiveness of BiFORM® Bioactive Moldable Matrix in an orthopedic (non-spine) application.
References:
- Chen, H.C. and Li, S.T. Evaluation of a Composite of Bioglass, Collagen, Anorganic Bone and Autograft in a Rabbit Spinal Fusion Model Society of Biomaterials, 2018.
- Hench, L.L., J Mater Sci: Mater Med (2006) 17: 967-978
- Xynos, I.D., A. J. Edgar, et al. (2000). Biochem Biophys Res Commun 276(2): 461-465
- Animal Study data on file at Collagen Matrix, Inc.
- ASTM F3207-17. Standard Guide for in In Vivo Evaluation of Rabbit Lumbar Intertransverse Process Spinal Fusion Model.
- Matsuura, A., Kubo, T., Doi K., Hayashi, K., Morita, K., Yokota, R., Hayashi, H., Hirata, I., Okazaki, M., Akagawa, Y. (2009). Bone formation ability
of carbonate apatite-collagen scaffolds with different carbonate contents. Dental Materials Journal, 28(2), 234-242. - Ellies, LG., Carter, J.M., Natiella, J.R., Featherstone, J.D.B., Nelson, D.G.A. (1988). Quantitative analysis of early in vivo tissue response to synthetic apatite implants. J. of Biomed. Mater. Res., 22, 137-148.
In Vitro Bioactivity Test

Vitoss BA Incubated in SBF from 0 to 120 hours.
I. Objective
The objective of the study was to evaluate the ability of the BiFORM® Bioactive Moldable Matrix to induce calcium phosphate layer formation using simulated body fluid as compared to Vitoss BA.
II. Materials & Methods
Both BiFORM® Bioactive Moldable Matrix and Vitoss BA were incubated in SBF for 5 days. Samples were then analyzed to determine the changes in chemical composition and surface morphology by field emission scanning electron microscopy (FESEM) and energy dispersive spectroscopy (EDS).
III. Results
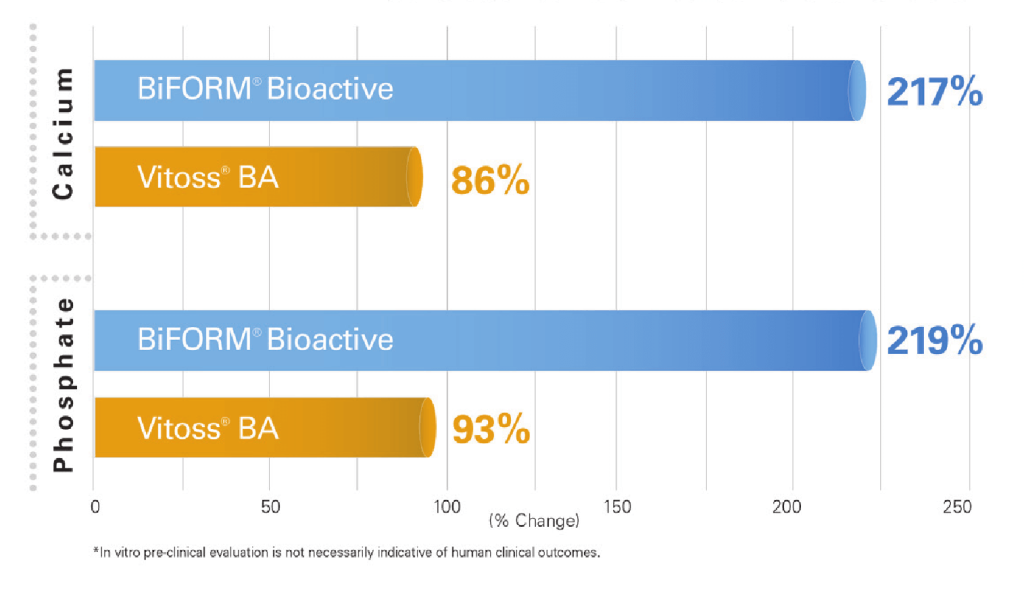
The bioactivity of BiFORM® Bioactive Moldable Matrix was demonstrated from the induction of calcium and phosphate growth widespread on the surface of the implant as shown in Figure 1. EDS analysis confirmed the increase in calcium and phosphate content after 5 days of incubation in SBF.
In addition, results from the in vitro bioactivity test demonstrate, BiFORM® Bioactive Moldable Matrix induces almost 2.5x more calcium phosphate deposition than Vitoss® BA1,2. The bioactivity testing was performed per ISO 23317 for apatite forming ability of implant materials as shown in Figure 2.
IV. Conclusion
FESEM images reveal apatite layer formation on both BiFORM® Bioactive Moldable Matrix and Vitoss BA after soaking in SBF for 5 days. EDS analysis confirms the increase in calcium and phosphate content for BiFORM® Bioactive Moldable Matrix.
References:
- In vitro data on file at Collagen Matrix, Inc.
- ISO23317 Implants for surgery – In vitro evaluation for apatite forming ability of implant materials.




