Compatibility Assessment of BIASURGE® Advanced Surgical Solution with Implantable Materials and Closure Technologies
Authors: Bradley Rodier, PhD, Rebecca McMahon, PhD, Nina Bionda, PhD
- Introduction
In the United States, Surgical Site Infections (SSIs), a major category of Hospital-Acquired Infections (HAIs), impose a significant economic burden on the healthcare system. It is estimated that they affect up to 300,000 patients and cost approximately $3.3 billion annualy.1, 2 SSIs are also associated with a markedly increased mortality risk, elevating it by 2 to 11 times, with 75% of deaths related to SSIs being directly attributed to the infection.1, 3, 4 BIASURGE® Advanced Surgical Solution is a no-rinse irrigation solution which contains a synergistic combination of EDTAs, PHMB, and vicinal diols,5 and designed to effectively prevent and reduce microbial contamination, thereby enhancing patient safety.
Given the nature of this product, BIASURGE has the potential to interact with a variety of materials that are crucial to the success of surgical procedures and patient outcomes. These materials may include prosthetics, surgical cement, or adhesives, and the patient’s tissue and associated fluids. Therefore, it is essential to test the compatibility of BIASURGE with these materials to ensure that it does not impair their function. This white paper presents the results of a series of tests examining BIASURGE compatibility with blood clotting, various surgical materials including bone cements, and surgical adhesives or sealants. Additionally, it investigates whether BIASURGE exhibits any corrosive or oxidative properties when applied to common prosthetic metals.
- Methodology
In vitro blood clot testing: Blood clot testing was performed to determine potential inhibition of blood clotting due to BIASURGE in comparison to a surgical wound cleanser containing benzalkonium chloride (Table 1). Commercially available sheep’s blood was treated with 0.5 M calcium chloride (CaCl2) solution to reverse the anti-coagulation. Varying amounts of the test article (BIASURGE or comparator) were then added to the prepared blood and the time to clot was measured and compared to clot time of blood to which no test articles were added. The amount of test article added to the blood varied from 1% to 15%, as shown in Table 1.
Bone cement compatibility testing: This testing was performed to ascertain BIASURGE compatibility with common biomaterials used in a variety of orthopedic procedures. The articles tested consisted of two surgical cements, a barium sulfate and polymethylmethacrylate (PMMA) composite and a calcium
sulfate-based cement (Table 2). The bone cements were tested in a simulated cavity, a 2 cm diameter polypropylene well. All products were prepared in accordance with instructions for use (IFU) and testing consisted of 3 parts:
Pre-application: Before application of the bone cements, the wells were treated with BIASURGE, the treatment aspirated, followed by application of the cement to the simulated cavity.
Post application: The test article was applied to the simulated cavity and allowed to react as indicated in the IFU. Once fully reacted, the cements were washed with 60 mL of BIASURGE. In
both of these scenarios, the mechanical properties of the cement were tested via application of sheer forces to the material’s surface and impact testing, when applicable, to determine delamination and/or material loss (visual inspection).
Solubility: The fully formed product was submerged in 60 mL of BIASURGE and allowed to soak for at least 5 hours. The product was then inspected, dried, and weighed to determine any loss.
Adhesive compatibility testing: This testing was performed to ascertain BIASURGE compatibility with common surgical adhesives and sealants. Two materials were evaluated in this study, a synthetic cyanoacrylate surgical adhesive, and a fibrin surgical sealant (Table 2). The test substrates in this study were 2 x 4 cm pieces of porcine dermis heated to 37°C. All products were applied to the porcine dermis substrate in accordance with instructions for use (IFU) and tested similar to what was described in the bone cement methods: effect of BIASURGE pre-application, post-application, and solubility assessment.
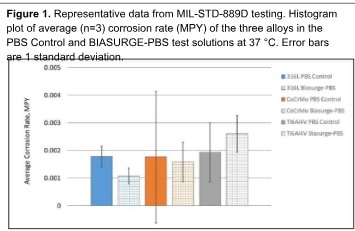
Corrosion testing: The corrosive effects of BIASURGE on common implant metals were performed using a panel of standard methods at physiological temperatures: ASTM F2129, MIL-STD-889D, and electrochemical impedance spectroscopy (EIS). The metals tested were a titanium aluminum vanadium alloy (Ti6Al4V), stainless steel (316L), and a cobalt-chromium-molybdenum alloy (CoCrMo). The general corrosion behavior, susceptibility crevice corrosion, and localized corrosion (i.e., pitting) of BIASURGE was compared to the control (buffered saline, PBS) using cyclic potentiodynamic polarization after soaking in the test article for 30 minutes. Report on file.6
- Results and Discussion
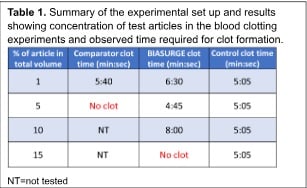
In vitro blood clot testing: The results for the clot testing can be found in Table 1. Blood diluted with BIASURGE formed a clot when diluted with up to 10% v/v with BIASURGE while comparator, benzalkonium chloride-based irrigation solution, resulted in failure to clot at concentrations above 1%. These results indicate that BIASURGE does not inhibit clotting of the blood in vitro. Though it does appear that BIASURGE slightly slowed down the speed of the clotting
process, when used at higher concentrations (i.e., 10%), these levels of blood dilution are beyond of what is expected to occur in standard surgical procedures.
Bone cement and adhesive/sealant compatibility testing: The summary of the results for the compatibility testing for several commonly used bone cements and adhesives can be found in Table 2. BIASURGE had no solubilizing effect on any of the products tested. BIASURGE, when administered after the application of the test products, did not alter the properties of either type of bone cement or the cyanoacrylate-based adhesive. When the substrate was pre-treated with BIASURGE, this also had no impact on the application or final properties of either the barium sulfate/PMMA cement or the cyanoacrylate-based adhesive. The calcium sulfate-based cement did lose some material to the compression cloth when the cavity was pretreated with BIASURGE, this can be attributed to residual moisture in the cavity and a lack of a synthetic binder as in the case with the cyanoacrylate adhesive and barium sulfate/PMMA-based cement. Therefore, the small amount of residual moisture from BIASURGE provided enough mobility of the cement granules to be displaced. However, only a small percentage of material (<10%) was lost. The fibrin sealant showed some delamination from the porcine dermis both with pre- and post-application of BIASURGE. This can be attributed to the nature of the sealant itself.
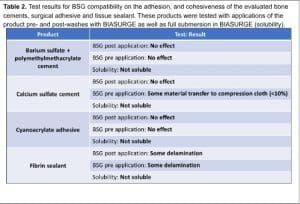
Fibrin sealants mimic natural clotting cascades and are sensitive to moisture at the point of application. In both cases, pre- and post-treatment with BIASURGE, additional moisture was added to the site of application which could inhibit the adhesion to the substrate surface. Of note, BIASURGE did not inhibit the fibrin sealant chemistry, e.g., clotting, just lowered its adhesive characteristic.
Corrosion testing: The corrosion behaviors of three different alloys were assessed under exposure to BIASURGE and a PBS control solution. Initial findings from the ASTM F2129 tests showed that, if exposed to BIASURGE solution, all three alloys experienced lower corrosion rates compared to samples immersed in the PBS control, with none showing susceptibility to crevice corrosion. MIL-STD-889D test results also indicated that immersing the alloys in BIASURGE for 30 minutes followed by PBS did not significantly differ in corrosion rate from immersion in PBS alone (Figure 1). However, Electrochemical Impedance Spectroscopy (EIS) measurements revealed varied responses for the different materials: while the 316L and CoCrMo alloys demonstrated a reduction in corrosion resistance after up to 30 minutes in BIASURGE compared to PBS, the Ti6Al4V alloy showed an increase in resistance under the same
conditions, which suggests the alloy surfaces had not achieved equilibrium with the test solutions within the exposure period. Overall, it should be noted that the average values and standard deviations of the values for the three alloys in the PBS and BIASURGE indicate there is no significant difference in corrosion behavior over the 30-minute exposure period.6
- Conclusion
The findings of this preclinical study underscore the compatibility of BIASURGE with various implantable materials and closure technologies.
BIASURGE showed no impact on blood clotting in vitro at concentrations up to 10%, which is above of what is expected to be present in contact with blood in a typical surgical procedure. Furthermore, BIASURGE did not solubilize any of the studied cements, sealants,
or adhesives and had a minimal effect on their functionality, except for the adhesiveness of fibrin sealants. As discussed above, the ability to coagulate was not affected and this reduction in adhesiveness can be attributed to the introduction of additional moisture to the sealant at the application site which reduced the adhesiveness of the water-based fibrin. Additionally, BIASURGE exhibited no corrosive or oxidative effects on common orthopedic metals when compared to the buffer control. These results confirm that BIASURGE® Advanced Surgical Solution is not expected to compromise performance of these materials. Additional investigations are needed to ascertain the translation of this preclinical data and comprehend its clinical implications.
References:
- Ban KA, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg. 2017; 224(1): 59-74.
- Zimlichman E, et al. Health Care–Associated Infections: A Meta-analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern Med. 2013; 173(22): 2039–2046.
- CDC, National Healthcare Safety Network: Surgical Site Infection Event (SSI), January 2023.
- Awad SS. Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg Infect (Larchmt). 2012; 13(4): 234-7.
- Salamone AB, et al. Synergistic Effect and Antibiofilm Activity of a Skin and Wound Cleanser. Wounds. 2020; 32(8): 208-216.
- UDR-TR-2022-159: Corrosion Susceptibility and Corrosion Rate Testing of Three Biomedical Alloys as a Function of Exposure to Proprietary Lavage Solution, 2023.




