Comparison of the Rapid Bactericidal Efficacy of Commercially Available Surgical Solutions

Authors: Larry Estlack, Rebecca McMahon, PhD, Nina Bionda, PhD
- Introduction
Intraoperative irrigation is a commonly used method aimed at reducing microbial burden by removing tissue debris, metabolic waste, and tissue exudate from the surgical field prior to site closure.1 With the absence of universally established standard of care and robust clinical data, the selection of appropriate irrigation solution remains a topic of debate. Isotonic saline has long been considered a safe and cost- effective irrigation solution and is most broadly used. Besides saline, the only other irrigation solution currently supported by the American Academy of Orthopaedic Surgeons, the Centers for Disease Control and Prevention, and World Health Organization is povidone-iodine.2-3
Reported here is a comparative assessment of the in vitro rapid bactericidal efficacy of a panel of nine commercially available irrigation solutions using short (60 seconds) contact time. The study was performed using two representative species of bacteria – Gram-positive methicillin-resistant Staphylococcus aureus (MRSA) and Gram-negative Pseudomonas aeruginosa. The irrigation solutions evaluated in the study involved an array of antimicrobial technologies and preservatives, including acids, surfactants, chlorhexidine gluconate (CHG), benzalkonium chloride (BZK), polyhexamethylene biguanide (PHMB) alone and in synergy with other additives, and povidone iodine (PI).
- Methodology
Briefly, aliquots of the different irrigation solutions were placed in sterile containers followed by the addition of the microbial inoculum (approximately 107 CFU/mL) and thorough mixing. After 60 second contact time, aliquots of the irrigation solutions were taken out, neutralized, and surviving bacteria enumerated through serial dilution and agar plating. The testing was performed against two representative bacteria, Gram-positive S. aureus, a methicillin-resistant strain (MRSA), and Gram-negative P. aeruginosa.
- Results and Discussion
The results are shown in Figure 1 and demonstrate rapid and complete bactericidal effect with the synergistic PHMB-based technology4 in BIASURGE® Advanced Surgical Solution as well as benzalkonium chloride (BZK)-containing irrigation solution. The remaining irrigation solutions showed varying level of efficacy with triple antibiotic solution having no detectable effectiveness, as expected given the short treatment time.
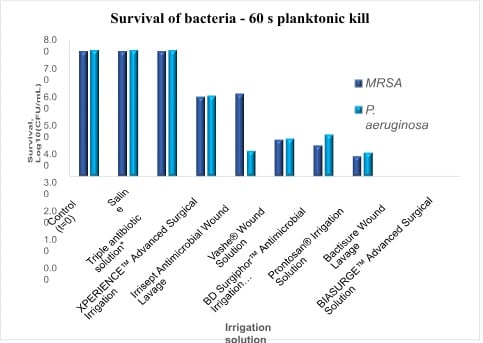
Further investigation, including in vivo and clinical studies, is needed to translate these findings and identify the irrigation solution with desired balance of low cytotoxicity and optimal antimicrobial properties.
References:
- Edmiston CE Jr, Spencer M, Leaper D. Antiseptic Irrigation as an Effective Interventional Strategy for Reducing the Risk of Surgical Site Infections. Surg Infect (Larchmt) 2018; 19: 774– 780.
- Goswami K, Austin MS. Intraoperative povidone-iodine irrigation for infection prevention. Arthroplast Today. 2019; 5(3): 306-308.
- Austin MS, Goswami K, Fleischman A, Parvizi J. A decade of protocol developments for SSI prevention: intraoperative betadine irrigation prevails. American Association of Hip and Knee Surgeons Annual Meeting. Dallas, TX. 2017.
- Salamone AB, et al. Synergistic Effect and Antibiofilm Activity of a Skin and Wound Cleanser. Wounds. 2020; 32(8): 208-216.
- Adams WP, Jr, Conner WC, Barton FE, Jr, et al. Optimizing breast-pocket irrigation: the post- betadine era. Plast Reconstr Surg. 2001; 107: 1596–1601.




