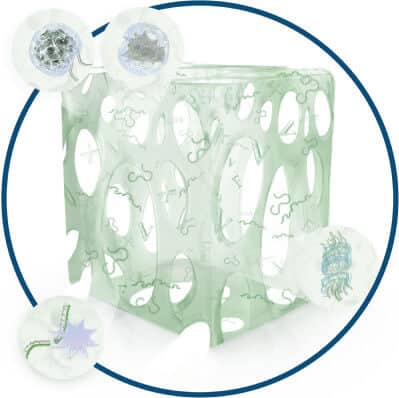
Eliminate and Prevent
BIASURGE® Advanced Surgical Solution eliminates 99.9999% of bacteria in 60 seconds in the solution and retains microbicidal activity for at least 24 hours1 after opening.
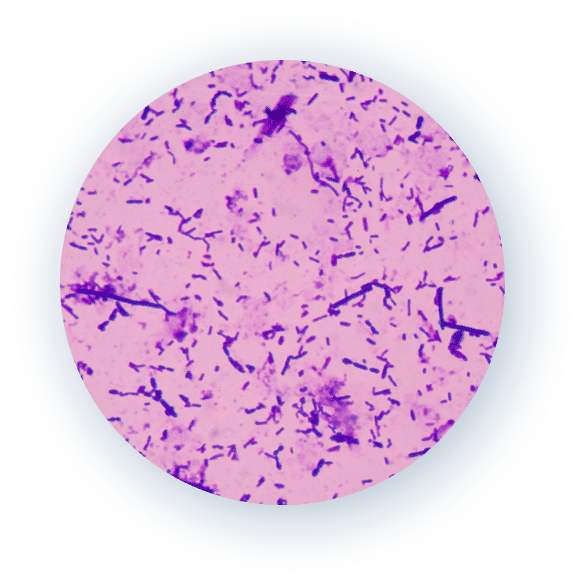
Research Studies
Research Study
In Vitro Testing
of
Various
Implant Materials
BIASURGE® Advanced Surgical Solution both eliminates biofilm-producing microbes and prevents them from attaching to the implant materials2
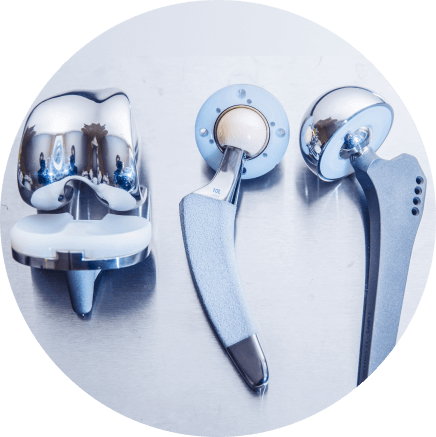
Research Study
Ex Vivo Testing of
Dermal Tissue
BIASURGE® Advanced Surgical Solution both eliminates biofilm-producing microbes and prevents them from attaching to porcine dermal tissue4

Research Studies:
- Data on file.
- Data on file.
- Salamone AB, Salamone JC, McMahon RE, Poleon S, Bionda N, D'Arpa P. Synergistic Effect and Antibiofilm Activity of a Skin and Wound Cleanser. Wounds. 2020 Aug;32(8):208-216. Epub 2020 May 7. PMID: 32804659.
- Data on file.
*Pre-clinical Study results may not directly correlate to human clinical outcomes.
MKT.15.17 Rev. 250217
Contact Sanara MedTech
"*" indicates required fields
Most Recent Press Releases
Sanara MedTech Inc. Announces Chief Executive Officer Transition
September 2, 2025