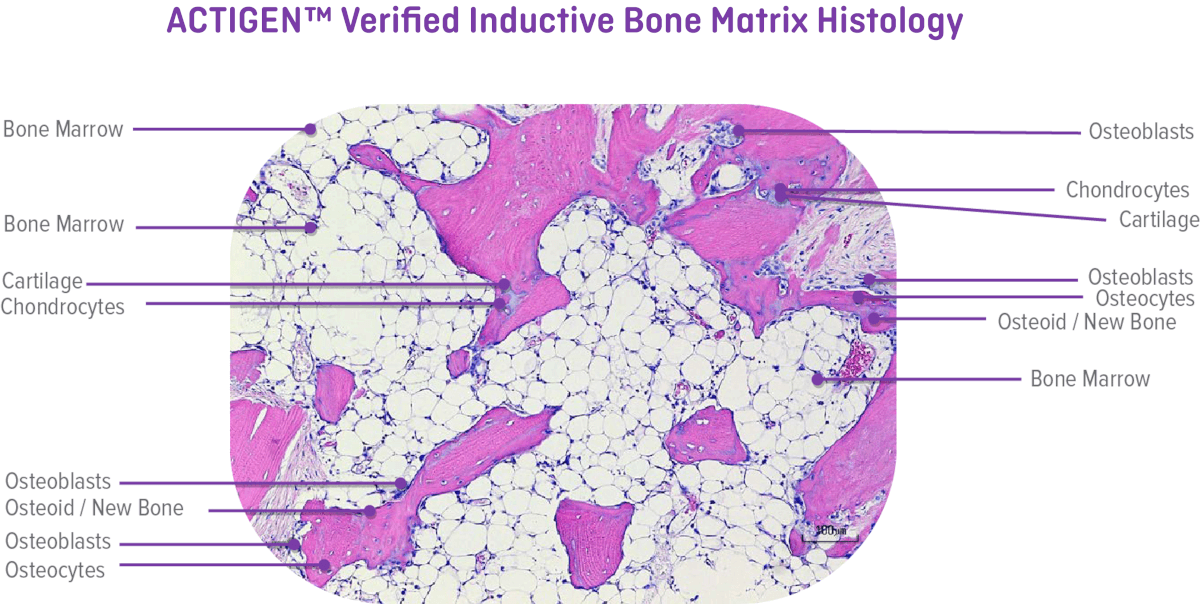
Histologically, ACTIGEN™ Verified Inductive Bone Matrix demonstrated the presence of all 5 elements of bone formation including new bone, bone marrow, osteocytes, chondrocytes, and cartilage in the athymic rat post-implantation at 28 days. In vivo testing is performed by an independent laboratory on every lot post-sterilization.
Contact Sanara MedTech
"*" indicates required fields
Most Recent Press Releases
Sanara MedTech Inc. Announces Chief Executive Officer Transition
September 2, 2025